After first claiming an FDA clearance last year for its mixed reality brain surgery navigation platform, Zeta Surgical has expanded its range with another agency clearance ahead of its planned U.S. launch.
This month, the company’s “GPS-like” approach received a 510(k) clearance to enable its use with additional instruments as well as integrate with hospital IT systems.
“Our vision is to ensure that advanced image-guided technology is no longer confined to specialized operating rooms or surgical suites,” Zeta Surgical Chief Operating Officer Hieu Le Mau said in a statement. “The addition of new surgical kits substantially broadens the system's applications, opening up new possibilities in neurosurgical procedures, while the enhanced connectivity with hospital systems allows surgeons to easily transfer and access patient information.”
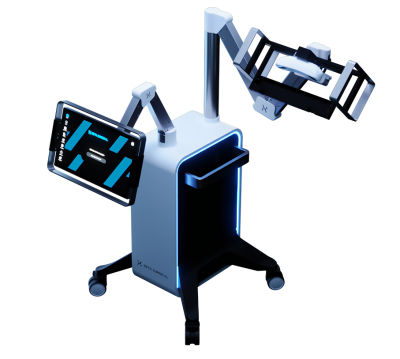
Earlier this year, the company reported that it had successfully completed its first clinical study, with the Zeta Cranial Navigation System being used at two sites in Singapore to treat 15 patients during elective and emergency ventriculostomies, or surgeries that place a catheter to drain the brain’s cerebrospinal fluid and monitor intracranial pressure.
Using computer vision and artificial intelligence software, the platform builds real-time visual overlays. However, instead of headset-based virtual reality, Zeta’s system relies on standard screens to track the placement of surgical tools.
The company said the latest FDA clearance coincides with the start of its commercial pilot program in the U.S., which aims to evaluate the Zeta system among different clinical applications. The limited market release is set to evolve into a broader launch in 2025.
“Our goal has always been to improve surgical outcomes, and this clearance opens up a significantly broader range of procedures that our partner hospitals can access through our minimally invasive system,” said Zeta Surgical’s co-founder and chief technology officer, Raahil Sha.